A Life Transformed: Claire’s Story
When Claire was just one year old, she could not speak or feed herself. Her parents had noticed troubling signs. While other babies her age were crawling and standing, Claire could not lift herself off her belly. The diagnosis came as a devastating blow. It was spinal muscular atrophy (SMA), a genetic disease that causes progressive muscle weakness. In its most severe forms, it is fatal within the first two years of life.
But Claire’s story did not end there. At the age of three, she was able to crawl, stand, and walk in a swimming pool. She talks, can yell, sing, and push herself in a manual wheelchair. What changed? A breakthrough treatment called Spinraza (nusinersen), one of the first antisense oligonucleotide (ASO) therapies approved for neurological disease.
Claire’s transformation represents one of many success stories emerging from a revolutionary class of precision medicines called antisense oligonucleotides. These synthetic DNA molecules are designed to target the genetic root of diseases, offering hope where conventional treatments have failed.
The Promise of Precision Medicine
Antisense oligonucleotides are short, synthetic strands of modified DNA or RNA. They are, typically just 15 to 30 nucleotides long, and they bind to specific RNA sequences in our cells. Think of them as molecular “spell-checkers” that can find and fix errors in our genetic instructions. Unlike traditional drugs that target proteins, ASOs work upstream, intervening at the RNA level before problematic proteins are even made.
Spinraza: The Breakthrough Drug
Spinraza’s approval in December 2016 marked an important moment for ASO therapy. In clinical trials with infants suffering from Type 1 SMA—the most severe form—the results were nothing short of remarkable:
- 51% of infants treated with Spinraza achieved motor milestones, compared to 0% in the control group
- Significant reduction in death or need for permanent ventilation (39% vs. 68%)
- Some children achieved the ability to sit unassisted, stand, or even walk, milestones they would never have reached without treatment
In older children with Type 2 SMA, Spinraza produced meaningful improvements in overall motor function, measured by standardized scales that assess movement capabilities. The drug has now been used to treat more than 14,000 patients worldwide across all age groups (1 day to 85 years of age), from infants to adults.
Beyond SMA: Expanding Applications
Following Spinraza’s success, additional ASO therapies have received approval:

The field has also witnessed groundbreaking personalized approaches. In 2019, researchers developed “milasen,” a custom ASO designed for a single patient—a six-year-old girl named Mila with a rare form of Batten disease. Created in less than a year, this personalized therapy slowed her disease progression, opening new possibilities for ultra-rare genetic conditions.
The Delivery Challenge: Reaching the Right Targets
Not unlike other gene therapies, the most challenging part is the delivery of treatment. To be effective treatment needs to be delivered where it is needed. For non-neurological conditions, an intravenous infusion (IV) is used or subcutaneous injections (SC). However, for neurological diseases ASOs are injected directly into the cerebrospinal fluid. This is carried out by a procedure called intrathecal injection, essentially a spinal tap. The procedure involves:
- Inserting a needle into the lower back
- Threading it between vertebrae into the spinal canal
- Injecting the drug directly into the fluid surrounding the spinal cord
This invasive approach is necessary because ASOs cannot readily cross the blood-brain barrier (BBB), the protective shield that keeps most substances out of the brain.
For Spinraza patients, this means four loading doses over the first two months at weeks 0, 2, 4, and 9. In addition, there is an ongoing maintenance dose every four months for life. In case of Tofersen, maintenance is indicated every 28 days. While there are obvious benefits of the treatment
Why the Blood-Brain Barrier is So Difficult
The blood-brain barrier is remarkably selective, allowing only small lipophilic molecules (under 400 Daltons) to pass freely. ASOs, by contrast, are large, charged molecules that the BBB treats as foreign invaders. Several factors make delivery particularly challenging:
- Size: ASOs are too large to pass through the tight junctions between brain capillary cells
- Charge: The negative charge on ASO backbones repels them from cell membranes
- Stability: Unmodified ASOs are rapidly degraded by enzymes in the bloodstream
Emerging Solutions
New approaches for ASO delivery include:
- Nanoparticle Delivery Systems – 45 nanometer glucose-coated particles bind to glucose transporters on brain blood vessels, essentially “piggybacking” across the barrier.
- Transferrin Receptor Targeting – “oligonucleotide transport vehicles” (OTVs) that bind to transferrin receptors on brain blood vessels.
- Neural Stem Cell Membranes – nanoparticles coated with membranes from neural stem cells, which naturally possess mechanisms to cross the BBB
- Chemical Modifications – are used to improve stability of ASOs and reduce both dosing frequency and toxicity. These include the following:
- Phosphorothioate backbones: Replace one oxygen atom with sulfur, increasing resistance to degradation
- 2′-O-methyl modifications: Enhance binding affinity to target RNA
- Locked nucleic acids (LNA): Create especially stable RNA-binding
Side Effects of ASOs – What Patients Need to Know?
While ASOs are generally well-tolerated, they’re not without risks. Understanding both common and serious side effects is crucial for patients and physicians. Some of side effects are related to the delivery procedure, e.g., the side effects of the intrathecal delivery. Other side effects are related to the ASOs themselves.
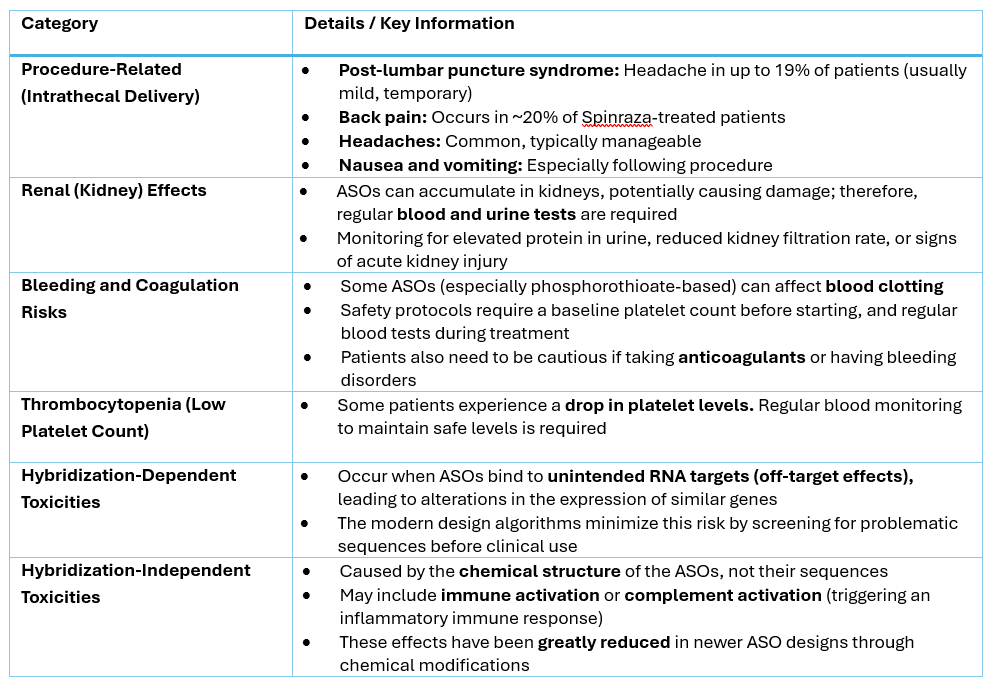
The Challenge of Clinical Efficacy
Perhaps the most sobering side effect is not the toxicity, but the lack of efficacy. Several high-profile ASO trials have shown disappointing results:
- BIIB078 for C9orf72 ALS: Successfully reduced toxic proteins but provided no clinical benefit, leading to discontinuation
- Tominersen: for Huntington’s Disease (HTT gene). Trial data showed failure to reduce mutant HTT in CSF in some groups and no meaningful functional benefit.
- BIIB105 for ALS: Reduced ataxin-2 protein but didn’t improve clinical function, resulting in program termination in 2024
These failures highlight a critical reality. Reducing a disease-causing protein does not always translate to clinical improvement, particularly if neuronal damage is already irreversible. The timing of the treatment is very important. While some of the treatments have been approved for adult patients, the most significant results are observed with early treatment.
Cost and Access: The $750,000 Question
No aspect of ASO therapy generates more controversy than cost. When Biogen announced Spinraza’s price in December 2016, it sparked immediate backlash. The price was $750,000 for the first year of treatment. Then it cost $375,000 annually thereafter for life. The cost-effectiveness debate is on-going, especially when money for coverage are not being increased by the governments.
The Institute for Clinical and Economic Review (ICER) is a drug-pricing watchdog organization. It concluded that Spinraza’s price “far exceeds common thresholds for cost-effectiveness.” Their analysis suggested more appropriate pricing would be $72,000 to $130,000 for the first year. Subsequent years cost should be between $36,000 to $65,000. However, Biogen and supporters argue that the current pricing reflects:
- Lack of an alternative. Without treatment, Type 1 SMA is typically fatal within two years, or survivors require permanent ventilation and 24/7 care. While this is true, there are alternative treatments available, for example Zolgensma
- Research investment. Decades of basic research and clinical trials
- Market size. Only about 10,000 people globally have SMA
- Comparable ultra-orphan drugs. Alexion’s Soliris costs over $500,000 annually
For comparison, the list price for Eteplirsen is approximately $300,000/year, and Viltolarsen – approximately $600,000/year. Pricing for other therapeutics is not widely available, and even those published are only approximate. These prices are not equal to what the patient pays out of pocket as the real cost depends on insurance, co-pay, manufacturer assistance or state/government programs. In many cases, pricing is confidential for newer therapies.
Access Barriers
The high cost creates significant access challenges associated with insurance coverage variability, geographic disparities and administrative burden.
| Category | Key Points |
|---|---|
| Insurance Coverage Variability | Most U.S. insurers cover Spinraza, but the approval process can be lengthy and complex. Requires prior authorization from insurance companies. Documentation of genetic confirmation of SMA Ongoing monitoring to justify continued treatment |
| Geographic Disparities | Access varies dramatically by country Some European countries negotiated confidential price reductions In developing nations, treatment largely unavailable despite WHO initiatives Even in wealthy countries, some health systems restrict access based on disease severity or age |
| Administrative Burden | Requires multiple specialists (neurologists, geneticists, physical therapists) Treatment centers with expertise in intrathecal administration Coordination between multiple healthcare providers and insurers |
Support Programs
Manufacturers offer assistance programs, though their scope varies. It can be co-pay assistance reducing out of pocket costs to $0. Some manufacturers provide patient assistance for uninsured or underinsured patients. The manufacturers also offer reimbursement support teams help to navigate insurance approval. Many families turn to platforms like GoFundMe to raise funds, highlighting the desperation these costs create. Patient advocacy groups like Cure SMA work to expand access globally, but progress is slow.
The bottom line is that we have treatments available for some of the rare diseases, but the global access is limited.
The Complex Reality of ASO Therapy
Antisense oligonucleotide therapies represent both a tremendous triumph and an ongoing challenge for modern medicine. Stories like Claire’s demonstrate the life-changing potential of precision genetic medicine. Children who would have died or been permanently ventilated are now walking, talking, and living fuller lives, and can become independent.
Yet the field must grapple with significant hurdles:
- Invasive delivery methods that place burden on patients
- Serious side effects requiring vigilant monitoring
- Astronomical costs that limit access
- Clinical trials showing that molecular success does not always equal clinical benefit
The future likely holds solutions to many of these challenges. Advances in BBB-crossing technologies could enable less invasive delivery. Improved manufacturing processes may reduce costs. Better biomarkers might help predict which patients will benefit most.
For now, ASO therapies represent what’s possible when scientific ingenuity meets medical need, imperfect, expensive, sometimes disappointing, but for families like Claire’s, nothing short of miraculous. As one parent said after their child received Spinraza: “We really just sat down and said we can’t allow this to just overtake us and be everything of every day. We just gotta live.”
That, ultimately, is what these therapies offer: the chance to live.
References:
- Spinal Muscular Atrophy Treatment: Claire’s Story | Children’s Hospital of Philadelphia, accessed 11 November 2025
- Lainey’s Spinal Muscular Atrophy Journey, accessed 11 November 2025
- High Cost Of Drug Spinraza A Barrier For Some In Need : Shots – Health News : NPR, accessed 11 November 2025
- How SPINRAZA® (nusinersen) Works, accessed 11 November 2025
- EXONDYS 51 (eteplirsen) | Treatment of Duchenne Muscular Dystrophy, accessed 11 November 2025
- VYONDYS 53 (golodirsen) | Duchenne Muscular Dystrophy Treatment, accessed 11 November 2025
- Exon 53-Skipping Therapy | Duchenne Muscular Dystrophy Treatment | VILTEPSO, accessed 11 November 2025
- AMONDYS 45 (casimersen), accessed 11 November 2025
- Home | Tegsedi, accessed 11 November 2025
- QALSODY® (tofersen)| Official Patient site, accessed 11 November 2025
- hATTR Neuropathy Diagnosis & Treatment Options | WAINUA® (eplontersen), accessed 11 November 2025
- Accelerated approval in US for DMD patients amenable to exon 53 skipping. 206488orig1s000ltr.pdf, accessed 11 November 2025
- FDA accelerated approval (DMD exon 53). Viltepso (viltolarsen) injection, accessed 11 November 2025
- FDA accelerated approval for DMD patients amenable to exon 45 skipping. Amondys 45, accessed 11 November 2025
- FDA approves treatment of amyotrophic lateral sclerosis associated with a mutation in the SOD1 gene | FDA, accessed 11 November 2025
- FDA accelerated approval of Wainua Approval Letter_217388Orig1s000ltr.pdf, accessed 11 November 2025
- Discontinued therapy BIIB078 not effective in ALS, trial data show, accessed 11 November 2025
- Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic – PMC, accessed 11 November 2025
- Biogen and Ionis Announce Topline Phase 1/2 Study Results of Investigational Drug in Amyotrophic Lateral Sclerosis | Biogen, accessed 11 November 2025
- ICER Issues Final Report and Policy Recommendations Regarding Treatments for Duchenne Muscular Dystrophy – ICER, accessed 11 November 2025
- ICER Issues Final Report on Spinraza and Zolgensma, Provides Policy Recommendations Related to Pricing and Coverage of Treatments for Spinal Muscular Atrophy – ICER, accessed 11 November 2025
- Cost Comparison – Pharmacoeconomic Review Report: Nusinersen (Spinraza) – NCBI Bookshelf, accessed 11 November 2025
- Biogen’s $375K Spinraza price puts a Sovaldi-style spotlight on rare disease meds | Fierce Pharma, accessed 11 November 2025
- Min HS, et al (2020) Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew Chem Int Ed Engl. 2020 May 18;59(21):8173-8180. doi: 10.1002/anie.201914751. Epub 2020 Mar 6. PMID: 31995252; PMCID: PMC7317551. accessed 11 November 2025
- Wang Y, et al (2021) Delivering Antisense Oligonucleotides across the Blood-Brain Barrier by Tumor Cell-Derived Small Apoptotic Bodies. Adv Sci (Weinh). 2021 May 4;8(13):2004929. doi: 10.1002/advs.202004929. PMID: 34258157; PMCID: PMC8261483, accessed 11 November 2025
- Barker SJ, et al (2024) Targeting the transferrin receptor to transport antisense oligonucleotides across the mammalian blood-brain barrier. Sci Transl Med. 2024 Aug 14;16(760):eadi2245. doi: 10.1126/scitranslmed.adi2245, accessed 11 November 2025
- Mendonca MCP et al (2021) Advances in the Design of (Nano)Formulations for Delivery of Antisense Oligonucleotides and Small Interfering RNA: Focus on the Central Nervous System | Molecular Pharmaceutics, vol 18/issue 4, accessed 11 November 2025
Note: This article is for informational purposes only and should not substitute for medical advice from qualified healthcare professionals. Treatment decisions should be made in consultation with physicians familiar with individual patient circumstances.


Leave a comment