One family, two terrifying diagnoses and $2.1 Million drug
Baby Josephine was diagnosed at 6 months of age with spinal muscular atrophy type 1 (SMA 1). The disease slowly destroys the motor neurons controlling her muscles. Without treatment, children with SMA Type 1 typically lose the ability to sit, swallow, and breathe, with most not surviving beyond age two. Josephine’s parents lived one day at the time and seen the baby’s decline. This was 2010, there was no treatment that can stop the progression of the disease or cure it. Traditional medicine offered only supportive care—feeding tubes, breathing assistance, and physical therapy to slow the inevitable decline. No pills could provide the missing SMN1 gene that Josephine’s motor neurons desperately needed to survive.
When the parents were expecting the second baby, they were hoping for the best. Baby Evelyn, was unfortunately, diagnosed with the SMA type 1 just like her sister. However, it happened within the 1 month of her life. Her father found out online about the clinical trials at Nationwide Childrens Hospital in Columbus, Ohio. It turned out she was a good candidate and was enrolled in the clinical trial of a new drug, Zolgensma. Evelyn was treated at 2 months of age with a single dose of Zolgensma. The progress was visible within weeks as she was lifting her head while on her tummy. The last video update showed her at 4.5 years of age fully independent, walking dancing, swimming, riding a tricycle. She lives the life that seem impossible at the time of diagnosis.
The miracle treatment, Zolgensma, is a gene therapy using a modified virus to deliver a normal SMN1 gene to the motor neurons. The catch? At $2.1 million, it’s one of the most expensive medications ever created. It is also important to highlight that the drug is not a cure. It simply stops the progression of the disease. This is why the key to success is early treatment, before the disease takes too much toll on the body. thus far we cannot yet restore the neurons that died. Newborn screening for SMA is critical here to catch affected babies early so they can be effectively treated.
The Delivery Problem
While designing the treatment is relatively easy, delivering it to the target cells is not. The human body is extraordinarily good at keeping foreign substances out. A defense system that protects us from infections, but also blocks therapeutic interventions. The greatest challenge is to deliver the medication to the right cells, in the right amounts for the right duration.
The traditional delivery methods using pills or injections have many limitations, such as:
- Most medications cannot cross the blood-brain barrier to reach neurons
- Drugs get diluted throughout the body, requiring massive doses with significant side effects
- Proteins and DNA-based therapies are broken down by digestive enzymes or immune systems
- Targeting specific cell types is not possible, resulting in drugs affecting both healthy and diseased cells equally
Spinal muscular atrophy exemplifies traditional medicine’s delivery problem. The disease occurs when patients lack functional SMN1 genes, causing motor neurons to gradually die. The solution seems simple, provide healthy SMN1 genes, but delivery is extraordinarily complex:
- Limited access to target cells. Motor neurons are located deep within the spinal cord, protected by multiple barriers
- Oral medications cannot transport large genetic material like therapeutic genes
- IV drugs do not efficiently cross from blood into spinal cord tissue
- Direct spinal injection risks paralysis and cannot reach all affected neurons
None of the non-viral gene delivery methods worked for neurological conditions. These methods included naked DNA injections, chemical delivery systems, electroporation, gene guns, and bone marrow transplants. They caused too much tissue damage, had limited efficiency or were rapidly degraded. Viruses solved the delivery problem because they’ve evolved specifically to:
- Penetrate cellular defenses by bypassing immune barriers that block other treatments
- Target specific cell types, different viruses naturally infect different tissues
- Deliver genetic cargo efficiently by transporting DNA directly into cell nuclei where genes are expressed
- Achieve long-term expression by integrating or persisting in cells for sustained therapeutic effect
Now, researchers have hijacked this natural viral machinery, removing harmful viral genes and replacing them with therapeutic ones. Adeno-associated virus (AAV) emerged as the ideal delivery vehicle because it:
- It is non pathogenic. Does not cause disease in humans
- Shows tissue specificity. Different AAV serotypes naturally target muscles, neurons, liver, or eyes
- Provides stable expression. Delivers genes that continue producing therapeutic proteins for years
- Avoids integration. Does not permanently alter the host genome like some other viruses
Real Patients, Life-Changing Results
Evelyn’s treatment began with extensive genetic testing to confirm her SMA diagnosis and rule out other conditions. The Zolgensma infusion itself was a simple procedure. It was administered through a standard IV over 60 minutes in a pediatric ICU, with doctors monitoring for potential immune reactions. Three months post-treatment, Evelyn began holding her head independently and from 6 months on reached out normal development milestones.
The outcomes of the clinical trials showed that treatment results are amazing. Children who, if untreated, would die were surviving. They were gaining improved motor skills, with many achieving standard developmental milestones. The quality of life achieved because of treatment was previously unthinkable. However, it also became clear that there are some additional variables affecting the outcomes of the treatment:
- Disease type – SMA1 or SMA2, and in case of the latter the number of copies of partly functional SMN2 gene
- Time of treatment – it is clear that earlier intervention improves outcome due to less damage caused by the disease
- Zolgensma is not a treatment for every child. One of the tests pre-treatment determine the level of antibodies to AAV9 virus used to deliver SMA1 gene.
AAV treatments and their side effects
There are currently four FDA approved treatments using AAV therapies. While majority of the patients survived the therapy and improved, there were some death reported. Two fatalities occurred after treatment with Zolgensma. They were due to acute liver failure 6 to 7 weeks post infusion, following steroid tapering. One therapy unrelated death occurred following Hemgenix treatment. The cause was urosepsis and cardiogenic shock occurred 65 weeks post-dosing. In 2025, two deaths were reported after treatment with Elevidys for Duchenne muscular dystrophy (DMD). As a result, FDA requested that the labels reflect the risks of liver injury, failure and death.
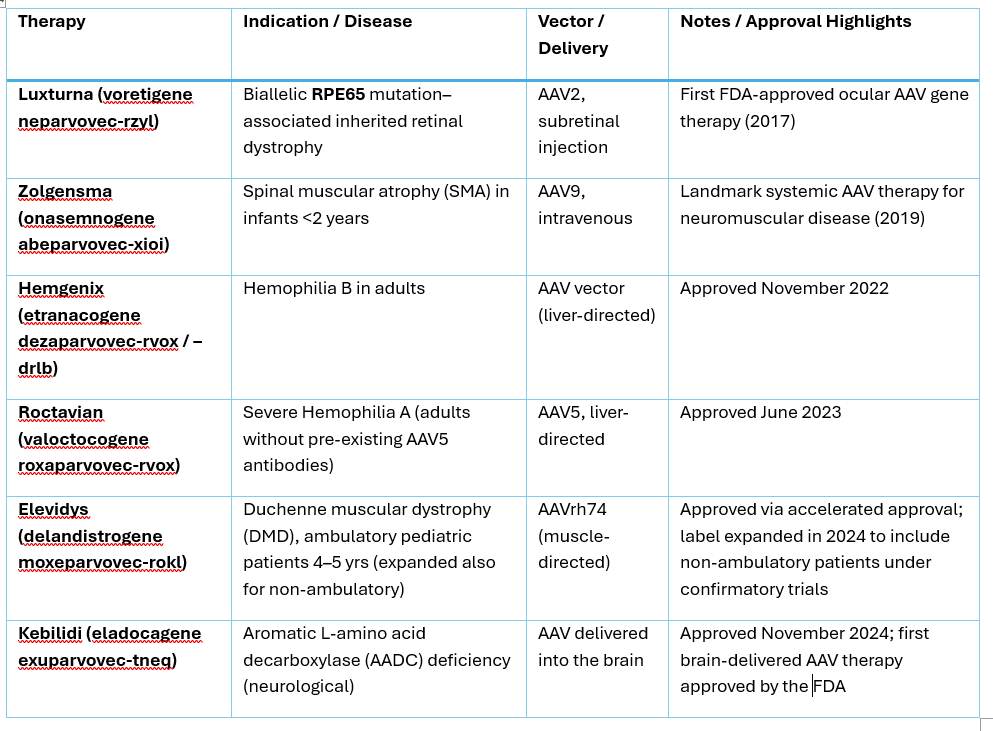
The most common side effects include immune reactions and liver injury or liver failure. Corticosteroids are used pre- and post-treatment to mitigate immune reactions. Liver enzymes are closely monitored post-treatment.
- Acute immune reactions to treatment. Patients develop fever, elevated liver enzymes and inflammation within days
- Pre-existing immunity to AAV can reduce efficacy or increase reaction risk
- High-dose AAV treatments have caused liver problems in some patients:
- Elevated liver enzymes. Occur in 20-30% of patients receiving high-dose treatments
- Hepatotoxicity, are rare cases of serious liver inflammation requiring intensive monitoring
The more concerning issue is gradual reduction in therapeutic protein levels over time. If real and ongoing, it can threaten the outcomes of the therapy in the long term. However, we do not know enough yet and ongoing patient followup is needed to determine this. If true, re-dosing can be problematic due to likely immune response.
The bottom line is evaluation of risks and benefits. These will be different for different diseases, however, risk-benefit ratio remains strongly favorable for approved indications. If you, your child, family member or a friend is considering any of the above treatments, discuss them in detail with your doctor. It is important to understand the risks and benefits of the therapy.
Cost and Access to treatments
As with the treatment for sickle cell disease, access to Zolgensma or any other AAV therapy can be problematic. Costs of treatments is very high, Hemgenix for treating hemophilia costs $3.5 Million per dose. Access in rich countries is possible, even for those with limited means. In developing countries, treatments like this can be out of reach due to healthcare infrastructure and economic constraints. However, patient assistance programs can be available locally through manufacturer programs, charitable foundations or access to clinical trials.
The future cost reductions are expected. As the competition increases, more efficient manufacturing methods are used and viral platforms can be used for multiple diseases.
A second issue is the need for specialized treatment centers. This is particularly true with treatments requiring surgical intervention for delivery, like brain infusion or a subretinal injection. There are likely to be on-going challenges with access equity to these treatments. The challenges arise because of the skill level required for delivery.
Looking into the future
Evelyn’s story represents thousands of patients whose lives have been transformed by viral delivery systems. Children with SMA are walking instead of requiring ventilators. Adults with inherited blindness are seeing their children’s faces clearly for the first time. Patients with bleeding disorders are living without the constant fear of life-threatening hemorrhages. The new chapter has been opened with in utero gene therapy for SMA. While still in its infancy it can be a major breakthrough for the future.
The therapeutic revolution has taught us that our assumptions about enemies and allies in biology may be fundamentally wrong. Sometimes the most powerful medicine comes not from fighting our microscopic adversaries, but from recruiting them as partners in healing. In the age of precision medicine, yesterday’s viral enemies have become today’s therapeutic heroes.
References:
- Spinal Muscular Atrophy (SMA) | Boston Children’s Hospital, accessed 8 Oct 2025
- Gene Therapy for SMA Type 1: Evelyn’s Story, accessed 8 Oct 2025
- Evelyn’s Story With ZOLGENSMA® (onasemnogene abeparvovec-xioi), accessed 8 Oct 2025
- FDA Approves First Gene Therapy Treatment for SMA Developed at the Abigail Wexner Research Institute at Nationwide Children’s Hospital, accessed 8 Oct 2025
- Patient Dies After Receiving DMD Gene Therapy, accessed 8 Oct 2025
- Teen’s death following Sarepta DMD gene therapy underscores a risk seen for decades | PharmaVoice, accessed 8 Oct 2025
- Novartis reports two children died from acute liver failure after treatment with Zolgensma gene therapy | STAT, accessed 8 Oct 2025
- Borge, B et al (2025) Intra-amniotic antisense oligonucleotide treatment improves phenotypes in preclinical models of spinal muscular atrophy. Science Translational Medicine, Vol 17, Issue 798, DOI: 10.1126/scitranslmed.adv4656
- Novel In Utero Gene Therapy Holds Promise for Patients with Spinal Muscular Atrophy | Johns Hopkins Medicine, accessed 8 Oct 2025
- Tizzano, AF et al, (2025) In utero therapy for spinal muscular atrophy: closer to clinical translation, Brain, Volume 148, Issue 9, Pages 3043–3056, https://doi.org/10.1093/brain/awaf123
- News Article | Evelina London, accessed 8 Oct 2025
- Gene Therapy for Spinal Muscular Atrophy (SMA) | Children’s Hospital of Philadelphia, accessed 8 Oct 2025
- Best outcomes seen with early Zolgensma gene therapy in real life | SMA treatment most effective before symptoms or by age 8 months | SMA News Today, accessed 8 Oct 2025
- How ZOLGENSMA® (onasemnogene abeparvovec-xioi) Works, https://youtu.be/S1oBk9_KZ2U?si=VuJAHG-6wE85V0MA, accessed 8 Oct 2025
- Presymptomatic Clinical Study Results | ZOLGENSMA®, accessed 8 Oct 2025
- Patient stories, ZOLGENSMA® (onasemnogene abeparvovec-xioi) – YouTube, accessed 8 Oct 2025
- LUXTURNA® (voretigene neparvovec-rzyl) – Inherited Retinal Disease, accessed 8 Oct 2025
- HEMGENIX® (etranacogene dezaparvovec-drlb) | How HEMGENIX Works, accessed 8 Oct 2025
- ROCTAVIAN® | Gene Therapy Treatment for Hemophilia, accessed 8 Oct 2025
- About ELEVIDYS (delandistrogene moxeparvovec-rokl), accessed 8 Oct 2025
- Therapy for AADC Deficiency | KEBILIDI™ (eladocagene exuparvovec-tneq), accessed 9 Oct 2025


Leave a comment